WikiJournal Preprints/CT Scan
Types
Generations of CT
The design and development of CT scanners went through multiple phases, which are collectively referred to as "generations of CT."

The first-generation CT scanner, developed by Godfrey Hounsfield, also known as EMI scanner operated on the 'translate-rotate' principle. This system employed a pencil X-ray beam and two detectors, facilitating the acquisition of two views in the through-plane direction.[1] The linear translation mechanism enabled the acquisition of 160 rays while the rotational movement captured 180 projections at 1° intervals, resulting in 28,800 rays for linear measurements.[2] The CT scanner took about 4 minutes to acquire one slice and 15-20 minuntes to process the data.[3][4] The first prototype model was installed in South London at Atkinson Morley's Hospital, and on October 1, 1971 first patient was scanned.[5] The first commercial scanner was installed at Mayo Clinic in 1973.[4]

Following the initial development of the first-generation CT, EMI introduced "CT 1010" an enhanced scanner in 1975 that eliminated the need for a waterbag. This system also employed "translate-rotate" configuration, but featured an upgraded setup with 8 detectors spanning 3 degrees. This enhancement allowed for a 3-degree rotation increment and required only 60 translations, significantly reducing the scan time to just 1 minute. This innovative design was subsequently referred to as the second-generation CT[6]. Subsequently, the detector count increased to 30, covering a range of 10 degrees and reducing the scan time to 20 seconds, as seen in the EMI 5000 series.[5][7]
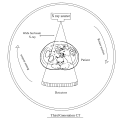
Third generation CT scanners used rotate-rotate configuration i.e. both the tube and the detectors rotated around the patient, employing a wide fan beam x-ray geometry and multiple detectors to collect the data.[8][9] In this generation of scanners, a singular detector element malfunction results in the erroneous recording of the corresponding ray in all projections, thereby inducing a ring artifact in the resultant images.[10] EMI 6000 belonged to the third generation of CT scanners.[11]

The Fourth-generation CT Scanners, initially pioneered by A.S.& E. Corporation,[12] employed detectors organized in a fixed ring comprising around 4800 individual detector elements. In this configuration, the X-ray tube generates a fan-beam X-ray and orbits around the patient. The ring artifact problem identified in Third-generation CT scanners was solved by this configuration. The EMI 7000 series similarly adhered to this principle.[13]
In Fifth generation CT, also know as electron beam computed tomography, both the x ray source and the detectors are stationary. This generation does not use conventional X-ray tube, rather employs a substantial tungsten arc (covering 210°) that surrounds the patient and directly faces the detector ring. An electron gun is utilized to guide and concentrate a rapid electron beam along the tungsten target ring within the gantry.[14] This type had a major advantage since sweep speeds can be much faster, allowing for less blurry imaging of moving structures, such as the heart and arteries.[15] Fewer scanners of this design have been produced when compared with spinning tube types, mainly due to the higher cost associated with building a much larger X-ray tube and detector array and limited anatomical coverage.[16]
Classification according to scanning method
Sequential CT, also known as step-and-shoot CT, is a scanning method in which the CT table moves stepwise. The process involves the table moving to a specific position, halting for the rotation and acquisition of a slice by the X-ray tube, followed by another incremental movement for the capture of subsequent slices. This method necessitates the table to pause during the slice acquisition, leading to increased scanning time due to interscan delays after each 360° rotation.[17] Before the introduction of slip ring technology, this scanning approach was commonly utilized. The need for the tube to return to its initial position after each rotation was essential to prevent cables connecting rotating components, such as the x-ray tube and detectors, from becoming entangled, resulting in prolonged interscan delays.

Spinning tube, commonly called spiral CT, or helical CT, is an imaging technique in which an entire X-ray tube is spun around the central axis of the area being scanned while the patient table is moving continuously.[18][19][20][21] Continuous scanning was made possible by slip ring technology. Slip rings provide an interface through a ring-and-brush arrangement, ensuring uninterrupted electrical connections. This eliminates the requirement for the X-ray tube to return to its initial position after each rotation, enabling continuous movement of the x-ray tube. The scanners with slip ring was introduced in 1987 by Siemens Medical Systems.[22]
Classifications according to the X-ray beam geometry
Pencil beam computed tomography employs a narrow, parallel X-ray beam geometry, while fan beam CT utilizes an X-ray beam that diverges outward from the radiation source.[23][12]
Cone beam computed tomography (CBCT) uses a diverging cone shaped x ray beam for the generation of images. This type is particularly well-suited for dentistry and orthodontics due to its ability to achieve high-resolution imaging with voxel sizes as small as 0.1 mm. Moreover, it offers a considerable advantage over spiral CT by utilizing substantially lower levels of radiation.[24]
Classifications according to the detectors
A Single-row CT scanner utilizes a solitary row of detectors, enabling it to gather data for a single slice. Consequently, it is also referred to as Single Slice CT. Multi-row detector CT scanners are equipped with multiple rows of detectors. These detector rows acquire images simultaneously, enabling the rapid acquisition of multiple slices at once. MSCT scanners offer enhanced image quality but come at the expense of increased radiation exposure compared to their single-slice CT. [25]
Photon-counting computed tomography is a recent advancement in computed tomography that employs a photon-counting detector to detect X-rays, registering the interactions of individual photons. Through monitoring the deposited energy in each interaction, the detectors capture an approximate energy spectrum.[26][27]
Flat Panel CT represents a CT scanner which is characterized by the integration of flat panel detectors. These scanners present a significant advancement in volumetric coverage, facilitating comprehensive imaging of entire organs such as the heart, kidneys, or brain through a singular axial scan. [28][29][29]
Dual Energy CT
Dual Energy CT also known as Spectral CT is an advancement of Computed Tomography in which two energies are used to create two sets of data.[30] A Dual Energy CT may employ Dual source, Single source with dual detector layer, Single source with energy switching methods to get two different sets of data.[31] It was commercially introduced in 2006.[32] Dual source CT is an advanced scanner with a two X-ray tube detector system, unlike conventional single tube systems.[33][34] These two detector systems are mounted on a single gantry at 90° in the same plane.[35] This scanner allow fast scanning with higher temporal resolution by acquiring a full CT slice in only half a rotation. Fast imaging reduces motion blurring at high heart rates and potentially allowing for shorter breath-hold time. This is particularly useful for ill patients having difficulty holding their breath or unable to take heart-rate lowering medication.[35][36]
Single Source with Energy switching is another mode of Dual energy CT in which a single tube is operated at two different energies by switching the energies frequently.[37][38] Dual layer spectral CT is a sub-type in which the spectral data is obtained by using two separate scintillator layers. It consists of two detector layer in which one is on the top of another. The detector layer that is closer to the x ray tube detects the low energy x rays and lets the high energy x rays to pass to the layer that is below. The high energy x rays are detected by the second layer.[39][40]
Hybrid CT imaging
Hybrid imaging involves integrating two or more imaging modalities to create a novel technique. This fusion leverages the inherent strengths of the combined imaging technologies, resulting in the emergence of a more potent and advanced modality.

Positron emission tomography–computed tomography is a hybrid CT modality which combines, in a single gantry, a positron emission tomography (PET) scanner and an x-ray computed tomography scanner, to acquire sequential images from both devices in the same session, which are combined into a single superposed (co-registered) image. Thus, functional imaging obtained by PET, which depicts the spatial distribution of metabolic or biochemical activity in the body can be more precisely aligned or correlated with anatomic imaging obtained by CT scanning.[41] PET-CT gives both anatomical and functional details of an organ under examination and is helpful in detecting different type of cancers.[42][43] Hybrid PET-CT systems have become more effective with the integration of anatomical details from CT scans. This integration allows for the creation of an attenuation correction map, which helps refine PET images. These advancements have notably reduced examination duration, increased diagnostic accuracy, and instilled greater confidence in the accuracy of diagnoses.[44][45] In oncology, studies show that using PET-CT together is better for accurately staging and restaging than using CT or PET alone.[46][47][48]
Single photon emission computed tomography- computed tomography also known as SPECT-CT is a hybrid imaging modality, used in Nuclear Medicine which combines a SPECT scanner and a CT scanner into one machine. This hybrid modality was first introduced commercially in 1999[49] SPECT-CT uses a radiotracer for evaluation of function details and x rays anatomical details. These image sets are then coregistered to allow for a comprehensive understanding of the relationship between physiological function and anatomical structures, aiding in more accurate and reliable diagnostic evaluations.[49]
Angio-CT is a hybrid machine which combines the fluoroscopic angiographic imaging and cross-sectional imaging of CT. These systems offer an integrated approach that combines the benefits of conventional angiography with the imaging capabilities of CT technology.[50] This hybrid imaging modality was introduced by Yasuaki Arai in 1992.[51][50]
Medical use
Since its introduction in the 1970s,[52] CT has become an important tool in medical imaging to supplement conventional X-ray imaging and medical ultrasonography. It has more recently been used for preventive medicine or screening for disease, for example, CT colonography for people with a high risk of colon cancer, or full-motion heart scans for people with a high risk of heart disease. The use of CT scans has increased dramatically over the last two decades in many countries.[53] An estimated 72 million scans were performed in the United States in 2007 and more than 80 million in 2015.[54][55]
Diagnostic
Head & Neck Imaging

CT scan remains the cornerstone imaging modality for the initial evaluation and subsequent management of patients with acute traumatic brain injury due to its rapid acquisition time and high sensitivity for detecting hemorrhagic complications, such as intraparenchymal hematomas and subdural hemorrhages.[56] CT scan of the head is typically used to detect infarction (stroke), tumors, calcifications, haemorrhage.[57] Tumors can be detected by the swelling and anatomical distortion they cause, or by surrounding edema. CT scanning of the head is also used in CT-guided stereotactic surgery and radiosurgery for treatment of intracranial tumors, arteriovenous malformations, and other surgically treatable conditions using a device known as the N-localizer.[58][59][60][61][62][63]
Contrast CT is generally the initial study of choice for neck masses in adults.[64] CT of the thyroid plays an important role in the evaluation of thyroid cancer.[65] CT scan often incidentally finds thyroid abnormalities, and so is often the preferred investigation modality for thyroid abnormalities.[65]
Body Imaging
A CT scan can be used for detecting both acute and chronic changes in the lung parenchyma, the tissue of the lungs.[66] It is particularly relevant here because normal two-dimensional X-rays do not show such defects. A variety of techniques are used, depending on the suspected abnormality. For evaluation of chronic interstitial processes such as emphysema, and fibrosis,[67] thin sections with high spatial frequency reconstructions are used; often scans are performed both on inspiration and expiration. This special technique is called high resolution CT that produces a sampling of the lung, and not continuous images.[68]
CT is an accurate technique for diagnosis of abdominal diseases like Crohn's disease,[69] GIT bleeding, and diagnosis and staging of cancer, as well as follow-up after cancer treatment to assess response.[70] It is commonly used to investigate acute abdominal pain.[71] Non-enhanced computed tomography is today the gold standard for diagnosing urinary stones.[72] The size, volume and density of stones can be estimated to help clinicians guide further treatment; size is especially important in predicting spontaneous passage of a stone.[73]
Musculoskeletal Imaging
CT scan is widely used for imaging of muscluloskeltal. For the axial skeleton and extremities, CT is often used to image complex fractures, especially ones around joints, because of its ability to reconstruct the area of interest in multiple planes.[74] Fractures, ligamentous injuries, and dislocations can easily be recognized with a 0.2 mm resolution.[75][76] With modern dual-energy CT scanners, new areas of use have been established, such as aiding in the diagnosis of gout.[77]
Perfusion Imaging

CT perfusion imaging is a specific form of CT to assess flow through blood vessels whilst injecting a contrast agent.[78] Blood flow, blood transit time, and organ blood volume, can all be calculated with reasonable sensitivity and specificity.[78] This type of CT may be used on the heart, although sensitivity and specificity for detecting abnormalities are still lower than for other forms of CT.[79] This may also be used on the brain, where CT perfusion imaging can often detect poor brain perfusion well before it is detected using a conventional spiral CT scan.[78][80] This is better for stroke diagnosis than other CT types.[80]
Cardiac Imaging

A CT scan of the heart is performed to gain knowledge about cardiac or coronary anatomy.[81] Traditionally, cardiac CT scans are used to detect, diagnose, or follow up coronary artery disease.[82] More recently CT has played a key role in the fast-evolving field of transcatheter structural heart interventions, more specifically in the transcatheter repair and replacement of heart valves.[83][84][85] The main forms of cardiac CT scanning are:
Coronary CT angiography (CCTA): the use of CT to assess the coronary arteries of the heart. The subject receives an intravenous injection of radiocontrast, and then the heart is scanned using a high-speed CT scanner, allowing radiologists to assess the extent of occlusion in the coronary arteries, usually to diagnose coronary artery disease.[86][87]
Coronary CT calcium scan: also used for the assessment of severity of coronary artery disease.[88] Specifically, it looks for calcium deposits in the coronary arteries that can narrow arteries and increase the risk of a heart attack.[89] A typical coronary CT calcium scan is done without the use of radiocontrast, but it can possibly be done from contrast-enhanced images as well.[90]
To better visualize the anatomy, post-processing of the images is common.[82] Most common are multiplanar reconstructions (MPR) and volume rendering. For more complex anatomies and procedures, such as heart valve interventions, a true 3D reconstruction or a 3D print is created based on these CT images to gain a deeper understanding.[91][92][93][94]
Interventional
CT-guided interventional procedures involve minimally invasive techniques guided by computed tomography imaging. These procedures utilize detailed cross-sectional images generated by CT scans to precisely guide various interventions. Common interventions performed under CT-guidance include biopsies for diagnostic purposes, drainage of fluid-filled areas, radiofrequency ablation to destroy tumors, and procedures like vertebroplasty or kyphoplasty for stabilizing fractured vertebrae.[95][96] The real-time imaging provided by CT ensures accuracy in needle or catheter placement during these procedures.[97][98] CT fluoroscopy proves to be a valuable clinical instrument, enhancing the efficiency of percutaneous abdominal and pelvic interventional procedures.[99]
CT-guided biopsies encompass guided imaging techniques, employing a CT scanner to direct the needle insertion during the procedure. These guided procedures can be either diagnostic or therapeutic.[100][101] CT-guided biopsies are widely utilized for diagnosing hepatic, renal, pulmonary, bone, pancreatic, adrenal, lymphatic, and brain lesions.[102][103]
CT-guided nephrostomies demonstrate feasibility and effectiveness, particularly in instances of iatrogenic ureteral injury.[104] This technique enables precise needle placement and the detection of subtle density variations within tissues[105][106] CT-guided percutaneous nephrostomy has proven to be efficient and safe, associated with low complication rates[107].
CT-guided tumor ablation involves using CT imaging for precise guidance during minimally invasive procedures to treat tumors. Techniques such as radiofrequency ablation and microwave ablation utilize heat to destroy cancerous tissues.[108][109] The visualization capabilities of CT enables to accurately target and ablate tumors, offering a less invasive alternative for patients with conditions such as liver tumors.[110]
Vascular Imaging
Computed tomography angiography (CTA) is a type of contrast CT to visualize the arteries and veins throughout the body.[111] This ranges from arteries serving the brain to those bringing blood to the lungs, kidneys, arms and legs. An example of this type of exam is CT pulmonary angiogram (CTPA) used to diagnose pulmonary embolism (PE). It employs computed tomography and an iodine-based contrast agent to obtain an image of the pulmonary arteries.[112][113]
Other uses
Industrial CT scanning (industrial computed tomography) is a process which utilizes X-ray equipment to produce 3D representations of components both externally and internally. Industrial CT scanning has been utilized in many areas of industry for internal inspection of components. Some of the key uses for CT scanning have been flaw detection, failure analysis, metrology, assembly analysis, image-based finite element methods[114] and reverse engineering applications. CT scanning is also employed in the imaging and conservation of museum artifacts.[115]
CT scanning has also found an application in transport security (predominantly airport security) where it is currently used in a materials analysis context for explosives detection CTX (explosive-detection device)[116][117][118][119] and is also under consideration for automated baggage/parcel security scanning using computer vision based object recognition algorithms that target the detection of specific threat items based on 3D appearance (e.g. guns, knives, liquid containers).[120][121][122]
X-ray CT is used in geological studies to quickly reveal materials inside a drill core.[123] Dense minerals such as pyrite and barite appear brighter and less dense components such as clay appear dull in CT images.[124]
X-ray CT and micro-CT can also be used for the conservation and preservation of objects of cultural heritage. For many fragile objects, direct research and observation can be damaging and can degrade the object over time. Using CT scans, conservators and researchers are able to determine the material composition of the objects they are exploring, such as the position of ink along the layers of a scroll, without any additional harm. After scanning these objects, computational methods can be employed to examine the insides of these objects.[125] Micro-CT has also proved useful for analyzing more recent artifacts such as still-sealed historic correspondence that employed the technique of letterlocking (complex folding and cuts) that provided a "tamper-evident locking mechanism".[126][127]
Procedure
Before starting the procedure, the patient preparation is necessary to ensure optimal scan quality and safety. This preparation includes a thorough examination of the patient's medical history to identify any potential contraindications. The patient is briefed about the procedure, and informed written consent is obtained from the patient on family member.

The specific preparation measures vary depending on the type of scan and the targeted organ. For abdominal or pelvic CT scans, fasting of 4-6 hrs is advised to minimize bowel gas interference and to reducing contrast reaction incidents.[2] However, recent studies indicate that prolonged fasting may be unnecessary for elective, non-gastrointestinal contrast-enhanced CT scans, particularly suggesting that fluid intake restrictions can be omitted.[1] Pre-scan instructions are also influenced by the use of contrast material, with some patients advised to refrain from certain medications, especially those affecting kidney function.[128][129]
Patients undergoing CT scans may experience anxiety, either due to the unfamiliar environment or, in some cases due to claustrophobia.[130] Maintaining stillness during the examination can be challenging for them. In such cases, a sedative can be used to alleviate the patient's anxiety and ensure an artifact free scanning process.[131]
Before the actual scan, a topogram, also known as a scout image or localizer image, is taken which is a low-dose, low-resolution radiographic image. This initial image helps to plan the coverage and orientation of the subsequent CT scan. The topogram provides a preliminary overview of the area to be imaged, allowing technologist to plan the scan.[132] Since, Topograms have a larger field of view than main scan, they can also play a role in revealing significant findings outside the scan field of view.[133]
Non-contrast CT

CT procedure in which contrast media is not used is often called as Non-Contrast CT (NCCT) or plain CT. This procedure is employed when there is already a sufficient contrast distinction in the target tissues, rendering the resulting image diagnostically significant. The process involves acquiring a topogram, followed by scanning the region of interest and reconstructing the data, marking the conclusion of the procedure. The non-contrast CT scans are rapid, less hazardous, and cost-effective procedures. Non-contrast CT head scans are useful in the identification of various conditions, encompassing traumatic hemorrhages, subdural hematomas, cerebral edema, fractures, and in detecting foreign bodies, such as tempered glass, in emergency situation.[134]
Contrast CT

Contrast CT or CECT procedures involves the use of a contrast medium for better visualization. Contrast media, also known as contrast agents, are substances used in imaging to improve the visibility of internal structures or fluids during diagnostic procedures. These agents enhance the differentiation between various tissues, and between normal and abnormal tissues allowing for clearer and more detailed imaging.[135] Contrast agents employed in CT imaging also know as Radio contrasts are generally categorized into Positive, Negative, and Neutral contrast agents. Positive contrast agents increase the x-ray attenuation and negative contrast agents reduce x-ray attenuation. Neutral contrast media, on the other hand, do not alter attenuation but are employed to enhance distention.[136]

Positive contrast agents can be categorized into Iodinated, Oily, or Barium sulfate contrast agents based on their composition. The most prevalent among them are Iodinated contrast agents, which are based on Iodine. These are further classified into Ionic contrast media and non-ionic contrast media.[137] The ionic iodinated contrast is further divided into Ionic monomers and Ionic dimers, & the non ionic iodinated contrast media is divided into non-ionic monomers and non-ionic dimers. Ionic monomers constitute a type of high-osmolar contrast media with an Iodine-to-particle ratio of 3:2, while Ionic dimers and Non-ionic monomers share an Iodine-to-particle ratio of 3:1. Non-ionic dimers, on the other hand, have a ratio of 6:1. The decrease in osmolarity is associated with the increase in the viscosity.[138]
Barium based contrast are used in the imaging of gastrointestinal tract. These contrast agents help outline the contours of the GI organs, such as the stomach and intestines, making them more visible on CT scans. Barium provides a high degree of contrast due to high x ray attenuation. It can also be used for double contrast studies eg in case of barium enema. It is water insoluble and is not absorbed by the gut.
Mainly Intravenous iodinated contrast media are used as positive contrast agents is used in CT, with some procedures Oral contrast and Negative contrast media can also be used. The use of contrast media mandates a thorough review of the patient's medical history and allergies, & assessment of renal function. Explicit written consent is imperative before administering the contrast material, delivered through an intravenous line in the arm or hand at a controlled rate via hand injection or a pressure injector. Upon injecting, the scan is initiated with a specific delay. This temporal coordination is importance for observing distinct phases of enhancement as the contrast moves to different organs.
Contrast Phases
Contrast phases refer to distinct stages in the enhancement of blood vessels or tissue after the administration of a intravenous contrast agent during a CT procedure. During a contrast-enhanced CT scan, various contrast phases delineate the dynamic enhancement of blood vessels. The early arterial phase manifests 15-20 seconds after injection of contrast followed by late arterial phase at 30-40 seconds post-injection. Subsequently, the portal venous phase unfolds 70-90 seconds after injection. The nephrogenic phase starts at 100-120 seconds post-injection. The excretory phase also called as washout phase occurs at 5-10 minutes after injection. A scan in which multiple phases are acquired is called as a multiphasic study.
Contrast injection techniques
Test bolus is a small, preliminary injection of contrast agent given to a patient before the actual CT scan. The purpose of the test bolus is to determine the optimal timing for the contrast-enhanced scan and in the mean time also assess the integrity of venous access before administering the complete bolus of contrast medium.[139]
Bolus tracking is a technique employed in CECT to monitor the concentration of contrast material within a designated region of interest, typically a blood vessel. A region of interest (ROI) is typically positioned just before the target organ, The scanning process starts when the contrast concentration attains a predefined HU level, ensuring optimal filling of blood vessels with contrast. This approach aids in acquiring images at the point of peak enhancements.[140]
Parts of CT Scanner
CT scanner comprises of multiple components that work together to produce an image. The design of CT scanners and their individual parts varies among different manufacturers and models. The primary components of a standard CT scanner are enclosed within a structure known as the gantry. This gantry serves as a protective cover for the internal mechanisms, which operate at high speeds. Many scanners permit the gantry to tilt up to 20 degrees, which can be advantageous for procedures requiring such an angle. The typical gantry aperture is 70 cm, allowing the table to pass through it during scanning.

T: X-ray tube
D: X-ray detectors
X: X-ray beam
R: Gantry rotation
X Ray Tube
An X-ray tube is a device used to produce X-rays.[141] It includes a cathode kept at a negative voltage. The filament at the cathode is heated by a filament current (measured in milliamps) to emit electrons. These electrons are then accelerated by a high voltage difference, typically between 80 to 140 kilovolts, between the cathode and anode. When the electrons collide with the anode material, X-rays are generated. However, 99% of the energy is transformed into heat, and only 1% is converted into X-rays. Since, CT operates at higher tube voltage and for longer time, the heat capacity of the ray tubes is higher.[142]
Detectors
Detectors are the x ray sensitive devices that detect and measure the intensity of the x rays that pass through the body of the patient. Radiation interacts with detector materials through ionization or excitation, depositing a small amounts of energy which needs to be amplified. Detectors process and store signals using electronic circuits, operating in pulse mode, where each interaction is processed individually, or current mode, where signals are averaged. Pulse mode requires separating interactions by a time interval known as dead time, whereas current mode integrates signals to provide a net current proportional to the dose rate, suitable for high interaction rates to avoid dead time losses. Detector efficiency is determined by geometric and intrinsic efficiency, measures the probability of detecting emitted particles or photons, with efficiency varying based on proximity to the source and detector design. Various detector designs have been used in computed tomography.
In earlier days of computed tomography gas filled detectors were used. These detectors, such as ionization chambers function when the x rays ionize the gas between electrodes, generating a measurable electrical currents which is then processed to create an image. Ionization chambers operate at a voltage where all liberated charge is collected. Ionization chambers are used in current mode for their wide voltage range and lack of dead time losses, measuring exposure rates, and can be filled with high atomic number gases to increase sensitivity for specific CT scans.
In the recent years, semi conductor detectors have been used as CT detectors. A semiconductor diode with a reverse bias voltage supply can detect x rays by exciting low-energy electrons in the depletion region to the conduction band, creating a momentary current and voltage signal. The energy required to create an electron-hole pair is 3 eV, compared to 34 eV in ion chambers, producing a larger voltage pulse proportional to the energy deposited. Semiconductors can function as spectrometers due to their rapid electron-hole movement and shorter pulse rise time.
Patient Table
The patient table of a CT scanner is constructed from a low-attenuation material, which allows X-rays to pass through it. This table serves to position the patient correctly for scanning and moves during the scan to incrementally cover different areas. Since the scanner has a limited detector coverage in the z-direction, the table's movement is crucial. The distance the table moves during a complete rotation of the gantry is known as the table pitch or detector pitch.
Mechanism

Computed tomography scanner operates by using an X-ray tube that generates X-rays and rotates around the patient; X-ray detectors are positioned on the opposite side of the the X-ray source.[144] As the X-rays pass through the patient, they are attenuated by various tissues according to their density.[145] Tissues with higher density attenuate more x-ray photons while tissues with low density attenuate less, this attenuation data is acquired by the detectors around the patient. A visual representation of the raw data obtained is called a sinogram, yet it is not sufficient for interpretation.[146] The term sinogram was introduced by Paul Edholm and Bertil Jacobson in 1975.[147]
Once the scan data has been acquired, it is then processed using a form of tomographic reconstruction, which produces a series of cross-sectional images.[148] A These cross-sectional images are made up of small units of pixels or voxels.[149]
A pixel is a two dimensional unit based on the matrix size and the field of view. Pixels in an image obtained by CT scanning are displayed in terms of relative radiodensity. The pixel itself is displayed according to the mean attenuation of the tissue(s) that it corresponds to on a scale from +3,071 (most attenuating) to −1,024 (least attenuating) on the Hounsfield scale. When the CT slice thickness is also factored in, the unit is known as a voxel, which is a three-dimensional unit.[149]
Water has an attenuation of 0 Hounsfield units (HU), while air is −1,000 HU, cancellous bone is typically +400 HU, and cranial bone can reach 2,000 HU or more (os temporale) and can cause artifacts. The attenuation of metallic implants depends on the atomic number of the element used: Titanium usually has an amount of +1000 HU, iron steel can completely block the X-ray and is, therefore, responsible for well-known line-artifacts in computed tomograms. Artifacts are caused by abrupt transitions between low- and high-density materials, which results in data values that exceed the dynamic range of the processing electronics.
Initially, the CT scanners generated images in only transverse (axial) anatomical plane, perpendicular to the long axis of the body. Modern scanners allow the scan data to be reformatted as images in other planes. Digital geometry processing can generate a three-dimensional image of an object inside the body from a series of two-dimensional radiographic images taken by rotation around a fixed axis.[150] These cross-sectional images are widely used for medical diagnosis and therapy.[151]
Interpretation of results
Presentation

The result of a CT scan is a volume of voxels, which may be presented to a human observer by various methods, which broadly fit into the following categories:
- Slices (of varying thickness). Thin slice is generally regarded as planes representing a thickness of less than 3 mm.[152][153] Thick slice is generally regarded as planes representing a thickness between 3 mm and 5 mm.[153][154]
- Projection, including maximum intensity projection[155] and average intensity projection
- Volume rendering (VR)[155]
Technically, all volume renderings become projections when viewed on a 2-dimensional display, making the distinction between projections and volume renderings a bit vague. The epitomes of volume rendering models feature a mix of for example coloring and shading in order to create realistic and observable representations.[156][157]
Two-dimensional CT images are conventionally rendered so that the view is as though looking up at it from the patient's feet. Hence, the left side of the image is to the patient's right and vice versa, while anterior in the image also is the patient's anterior and vice versa. This left-right interchange corresponds to the view that physicians generally have in reality when positioned in front of patients.[158]
Grayscale
Pixels in an image obtained by CT scanning are displayed in terms of relative radiodensity. The pixel itself is displayed according to the mean attenuation of the tissue(s) that it corresponds to on a scale from +3,071 (most attenuating) to −1,024 (least attenuating) on the Hounsfield scale. A pixel is a two dimensional unit based on the matrix size and the field of view. When the CT slice thickness is also factored in, the unit is known as a voxel, which is a three-dimensional unit.[159] Water has an attenuation of 0 Hounsfield units (HU), while air is −1,000 HU, cancellous bone is typically +400 HU, and cranial bone can reach 2,000 HU.[160] The attenuation of metallic implants depends on the atomic number of the element used: Titanium usually has an amount of +1000 HU, iron steel can completely block the X-ray and is, therefore, responsible for well-known line-artifacts in computed tomograms. Artifacts are caused by abrupt transitions between low- and high-density materials, which results in data values that exceed the dynamic range of the processing electronics.[161]
Windowing
CT data sets have a very high dynamic range which must be reduced for display or printing as human eye can only detect 700-900 shades of gray.[162] This is typically done via a process of "windowing", which maps a range (the "window") of pixel values to a grayscale ramp. For example, CT images of the brain are commonly viewed with a window extending from 0 HU to 80 HU. Pixel values of 0 and lower, are displayed as black; values of 80 and higher are displayed as white; values within the window are displayed as a grey intensity proportional to position within the window.[163] The window used for display must be matched to the X-ray density of the object of interest, in order to optimize the visible detail.[164] Window width and window level parameters are manipulated by the radiologist or technologist to control the windowing of a scan.[165]
Multiplanar reconstruction and projections

Multiplanar reconstruction also known as MPR is the process of converting data from one anatomical plane (usually transverse) to other planes. It can be used for thin slices as well as projections. Multiplanar reconstruction is possible as present CT scanners provide almost isotropic resolution.[166] MPR is used almost in every scan. The spine is frequently examined with it.[167] An image of the spine in axial plane can only show one vertebral bone at a time and cannot show its relation with other vertebral bones. By reformatting the data in other planes, visualization of the relative position can be achieved in sagittal and coronal plane.[168] New software allows the reconstruction of data in non-orthogonal (oblique) planes, which help in the visualization of organs which are not in orthogonal planes.[169] It is better suited for visualization of the anatomical structure of the bronchi as they do not lie orthogonal to the direction of the scan.[170]
Curved-plane reconstruction is performed mainly for the evaluation of vessels. This type of reconstruction helps to straighten the bends in a vessel, thereby helping to visualize a whole vessel in a single image or in multiple images. After a vessel has been "straightened", measurements such as cross-sectional area and length can be made. This is helpful in preoperative assessment of a surgical procedure.[171]
Maximum Intensity Projection (MIP)
Maximum Intensity Projection is a scan visualization technique which is used to highlight the highest intensity voxels along a specific projection path. In MIP, each pixel in the final image represents the maximum intensity encountered along a ray traced through the volume data. This method is particularly useful in angiography and vascular imaging, where it enhances the visualization of blood vessels by emphasizing the contrast between vessels and surrounding tissues. MIP projections are valuable for detecting abnormalities and assessing the vascular anatomy with greater clarity.[172]
Minimum Intensity Projection (MinIP)
Minimum Intensity Projection highlight the lowest intensity values along a specific projection path. MinIP is particularly beneficial in visualizing structures with low attenuation or density, such as airways in lung imaging. By emphasizing low-intensity features, MinIP can enhance the visibility of subtle details and abnormalities that might be overshadowed in other types of reconstructions. This technique is commonly employed in pulmonary studies to improve the assessment of bronchial structures and airway abnormalities.[173][174]
Average Intensity Projection
In Average Intensity Projection (AIP), the image is displaying the average attenuation of each voxel within the selected volume.[175] As the slice thickness increases, the image becomes smoother and more akin to conventional projectional radiography.[176] AIP is particularly useful for identifying internal structures of solid organs or the walls of hollow structures, such as intestines.
| Type of projection | Schematic illustration | Examples (10 mm slabs) | Description | Uses |
|---|---|---|---|---|
| Average intensity projection (AIP) | 
|
The average attenuation of each voxel is displayed. The image will get smoother as slice thickness increases. It will look more and more similar to conventional projectional radiography as slice thickness increases. | Useful for identifying the internal structures of a solid organ or the walls of hollow structures, such as intestines. | |
| Maximum intensity projection (MIP) | 
|
The voxel with the highest attenuation is displayed. Therefore, high-attenuating structures such as blood vessels filled with contrast media are enhanced. | Useful for angiographic studies and identification of pulmonary nodules. | |
| Minimum intensity projection (MinIP) | 
|
The voxel with the lowest attenuation is displayed. Therefore, low-attenuating structures such as air spaces are enhanced. | Useful for assessing the lung parenchyma.[173][178] |
Volume rendering

A threshold value of radiodensity is set by the operator (e.g., a level that corresponds to bone). With the help of edge detection image processing algorithms a 3D model can be constructed from the initial data and displayed on screen. Various thresholds can be used to get multiple models, each anatomical component such as muscle, bone and cartilage can be differentiated on the basis of different colours given to them. However, this mode of operation cannot show interior structures.[179]
Surface rendering is limited technique as it displays only the surfaces that meet a particular threshold density, and which are towards the viewer. However, In volume rendering, transparency, colours and shading are used which makes it easy to present a volume in a single image. For example, Pelvic bones could be displayed as semi-transparent, so that, even viewing at an oblique angle one part of the image does not hide another.[180]
Image quality
The image quality in computed tomography depends upon the fidelity with which the generated images faithfully represent the attenuation values of X-ray beams as they pass through body tissues, as manifested in the resulting CT image. Image quality encompasses the accurate replication of fine details (Spatial Resolution) and minute discrepancies in attenuation (Contrast Resolution) within the depicted image.
Dose versus image quality
An important issue within radiology today is how to reduce the radiation dose during CT examinations without compromising the image quality. In general, higher radiation doses result in higher-resolution images,[181] while lower doses lead to increased image noise and unsharp images. However, increased dosage raises the adverse side effects, including the risk of radiation-induced cancer – a four-phase abdominal CT gives the same radiation dose as 300 chest X-rays.[182] Several methods that can reduce the exposure to ionizing radiation during a CT scan exist.
- New software technology can significantly reduce the required radiation dose. New iterative tomographic reconstruction algorithms (e.g., iterative Sparse Asymptotic Minimum Variance) could offer super-resolution without requiring higher radiation dose.[183]
- Individualize the examination and adjust the radiation dose to the body type and body organ examined. Different body types and organs require different amounts of radiation.[184]
- Higher resolution is not always suitable, such as detection of small pulmonary masses.[185]
Artifacts
Although images produced by CT are generally faithful representations of the scanned volume, the technique is susceptible to a number of artifacts, such as the following:[150][186]

Streaks are often seen around materials that block most X-rays, such as metal or bone. Numerous factors contribute to these streaks: under sampling, photon starvation, motion, beam hardening, and Compton scatter. This type of artifact commonly occurs in the posterior fossa of the brain, or if there are metal implants. The streaks can be reduced using newer reconstruction techniques.[187] Approaches such as metal artifact reduction (MAR) can also reduce this artifact.[188][189] MAR techniques include spectral imaging, where CT images are taken with photons of different energy levels, and then synthesized into monochromatic images with special software such as GSI (Gemstone Spectral Imaging).[190]
Partial volume effect appears as "blurring" of edges. It is due to the scanner being unable to differentiate between a small amount of high-density material (e.g., bone) and a larger amount of lower density (e.g., cartilage).[191] The reconstruction assumes that the X-ray attenuation within each voxel is homogeneous; this may not be the case at sharp edges. This is most commonly seen in the z-direction (craniocaudal direction), due to the conventional use of highly anisotropic voxels, which have a much lower out-of-plane resolution, than in-plane resolution. This can be partially overcome by scanning using thinner slices, or an isotropic acquisition on a modern scanner.[192]
Ring artifact probably the most common mechanical artifact, the image of one or many "rings" appears within an image. They are usually caused by the variations in the response from individual elements in a two dimensional X-ray detector due to defect or miscalibration.[193] This phenomenon is more frequently encountered in third-generation CT scanners equipped with solid-state detectors. These artifacts manifest as complete circles in sequential scans, or partial rings in helical CT scans.[194][195] Ring artifacts can largely be reduced by intensity normalization, also referred to as flat field correction.[196] Remaining rings can be suppressed by a transformation to polar space, where they become linear stripes.[193] A comparative evaluation of ring artefact reduction on X-ray tomography images showed that the method of Sijbers and Postnov can effectively suppress ring artefacts.[197]

Noise appears as grain on the image and is caused by a low signal to noise ratio. This occurs more commonly when a thin slice thickness is used. It can also occur when the power supplied to the X-ray tube is insufficient to penetrate the anatomy.[198]
Windmill artifacts is seen as a streaking appearances which can occur when the detectors intersect the reconstruction plane. This can be reduced with filters or a reduction in pitch.[199][200]
Beam hardening artefact can give a "cupped appearance" when grayscale is visualized as height. It occurs because conventional sources, like X-ray tubes emit a polychromatic spectrum. Photons of higher photon energy levels are typically attenuated less. Because of this, the mean energy of the spectrum increases when passing the object, often described as getting "harder". This leads to an effect increasingly underestimating material thickness, if not corrected. Many algorithms exist to correct for this artifact. They can be divided in mono- and multi-material methods.[187][201][202]
Motion artifact refers to unwanted distortions of the images caused by patient motion which can be voluntary on involuntary during the scanning process.
Advantages
CT scan has several advantages over traditional two-dimensional medical radiography. First, CT eliminates the superimposition of images of structures outside the area of interest.[203] Second, CT scans have greater image resolution, enabling examination of finer details. CT can distinguish between tissues that differ in radiographic density by 1% or less.[204] Third, CT scanning enables multiplanar reformatted imaging: scan data can be visualized in the transverse (or axial), coronal, or sagittal plane, depending on the diagnostic task.[205] The improved resolution of CT has permitted the development of new investigations. For example, CT angiography avoids the invasive insertion of a catheter. CT scanning can perform a virtual colonoscopy with greater accuracy and less discomfort for the patient than a traditional colonoscopy.[206][207] Virtual colonography is far more accurate than a barium enema for detection of tumors and uses a lower radiation dose.[208]
CT is a moderate-to-high radiation diagnostic technique. The radiation dose for a particular examination depends on multiple factors: volume scanned, patient build, number and type of scan protocol, and desired resolution and image quality.[209] Two helical CT scanning parameters, tube current and pitch, can be adjusted easily and have a profound effect on radiation. CT scanning is more accurate than two-dimensional radiographs in evaluating anterior interbody fusion, although they may still over-read the extent of fusion.[210]
Adverse effects
Contrast reactions
The administration of contrast agents carries potential risks, as adverse reactions may occur. While most serious reactions are typically observed after intravascular injection, adverse effects may still manifest after oral or intra-cavitary administration, as some contrast medium molecules may be absorbed into the circulation. Contrast reactions reported in CT scans are classified into three severity levels: Mild, Moderate, and Severe Reactions.
| Type | Severity | Symptoms may include | Medical attention |
|---|---|---|---|
| Mild Reaction | Low | nausea, headache, vomiting, flushing, pruritus, and a metallic taste. | Generally not required, typically resolve on their own. |
| Moderate Reactions | Intermediate | urticaria, severe vomiting, facial edema, bronchospasm, laryngeal edema, and vasovagal attacks. | Treatment approach varies based on the specific symptoms. |
| Severe Reaction | High | respiratory arrest, cardiac arrest, pulmonary edema, convulsions, and cardiogenic shock. | Immediate medical attention is essential |
In the United States half of CT scans are contrast CTs using intravenously injected radiocontrast agents.[211] The most common reactions from these agents are mild, including nausea, vomiting, and an itching rash. Severe life-threatening reactions may rarely occur.[212] Overall reactions occur in 1 to 3% with nonionic contrast and 4 to 12% of people with ionic contrast.[213] Skin rashes may appear within a week to 3% of people.[212]
The old radiocontrast agents caused anaphylaxis in 1% of cases while the newer, low-osmolar agents cause reactions in 0.01–0.04% of cases.[212][214] Death occurs in about 2 to 30 people per 1,000,000 administrations, with newer agents being safer.[213][215] There is a higher risk of mortality in those who are female, elderly or in poor health, usually secondary to either anaphylaxis or acute kidney injury.[211]
The contrast agent may induce contrast-induced nephropathy.[216] This occurs in 2 to 7% of people who receive these agents, with greater risk in those who have pre-existing kidney failure,[216] pre-existing diabetes, or reduced intravascular volume. People with mild kidney impairment are usually advised to ensure full hydration for several hours before and after the injection. For moderate kidney failure, the use of iodinated contrast should be avoided; this may mean using an alternative technique instead of CT. Those with severe kidney failure requiring dialysis require less strict precautions, as their kidneys have so little function remaining that any further damage would not be noticeable and the dialysis will remove the contrast agent; it is normally recommended, however, to arrange dialysis as soon as possible following contrast administration to minimize any adverse effects of the contrast.
In addition to the use of intravenous contrast, orally administered contrast agents are frequently used when examining the abdomen.[217] These are frequently the same as the intravenous contrast agents, merely diluted to approximately 10% of the concentration. However, oral alternatives to iodinated contrast exist, such as very dilute (0.5–1% w/v) barium sulfate suspensions. Dilute barium sulfate has the advantage that it does not cause allergic-type reactions or kidney failure, but cannot be used in patients with suspected bowel perforation or suspected bowel injury, as leakage of barium sulfate from damaged bowel can cause fatal peritonitis.[218]
Side effects from contrast agents, administered intravenously in some CT scans, might impair kidney performance in patients with kidney disease, although this risk is now believed to be lower than previously thought.[219][216]
Extravasation
Extravasation refers to the unintended leakage of contrast medium, from the intravascular space into the surrounding tissues.[220] This phenomenon can occur due to various mechanisms during medical procedures involving injection of contrast medium. The fluid can seep into perivascular tissue either by extraluminal dislocation of cannula. Additionally, a leak may manifest at the puncture site of a properly positioned cannula, causing extravasation. The direct impact of pressure from the jet against the vessel wall can lead to vessel disruption and, consequently, extravasation.[221] Extravasation can arise from both manual hand injection and injections administered using a press-injector. Patients at a greater risk of experiencing extravasation encompass the elderly, infants, children, individuals with impaired consciousness, and those with pre-existing vascular conditions.[222]
Moderate extravasation is characterized by symptoms, such as skin blistering, progressive edema, or ulceration. Close monitoring is advised, and physician assessment is recommended to evaluate for potential neurovascular compromise. This assessment includes checking peripheral pulse and assessing sensation distal to the affected limb. Severe extravasation represents a critical condition with the potential for neurovascular compromise, signs of tissue necrosis, or compartment syndrome. Urgent surgical attention, specifically emergency fasciotomy, is necessary to alleviate pressure within the affected compartment and prevent further complications. [223]
Scan dose
| Examination | Typical effective dose (mSv) to the whole body |
Typical absorbed dose (mGy) to the organ in question |
|---|---|---|
| Annual background radiation | 2.4[224] | 2.4[224] |
| Chest X-ray | 0.02[225] | 0.01–0.15[226] |
| Head CT | 1–2[227] | 56[228] |
| Screening mammography | 0.4[229] | 3[230][226] |
| Abdominal CT | 8[225] | 14[228] |
| Chest CT | 5–7[227] | 13[228] |
| CT colonography | 6–11[227] | |
| Chest, abdomen and pelvis CT | 9.9[228] | 12[228] |
| Cardiac CT angiogram | 9–12[227] | 40–100[226] |
| Barium enema | 15[230] | 15[226] |
| Neonatal abdominal CT | 20[230] | 20[226] |
The magnitude of radiation exposure encountered by patients undergoing computed tomography examinations depends on the scanner's design,[231] and the factors chosen by the radiology technologist including current, voltage, scan field, scan duration, filtration, rotation angle, collimation, and section thickness and spacing, collectively contribute to the overall cumulative dose.[232][233] These considerations are important for optimizing both equipment configurations and scanning protocols to have a balance between achieving diagnostic precision and minimizing radiation exposure to patients.[234]
The table reports average radiation exposures; however, there can be a wide variation in radiation doses between similar scan types, where the highest dose could be as much as 22 times higher than the lowest dose.[227] A typical plain film X-ray involves radiation dose of 0.01 to 0.15 mGy, while a typical CT can involve 10–20 mGy for specific organs, and can go up to 80 mGy for certain specialized CT scans.[226]
For purposes of comparison, the world average dose rate from naturally occurring sources of background radiation is 2.4 mSv per year, equal for practical purposes in this application to 2.4 mGy per year.[224] While there is some variation, most people (99%) received less than 7 mSv per year as background radiation.[235] Medical imaging as of 2007 accounted for half of the radiation exposure of those in the United States with CT scans making up two thirds of this amount.[227] In the United Kingdom it accounts for 15% of radiation exposure.[229] The average radiation dose from medical sources is ≈0.6 mSv per person globally as of 2007.[227] Those in the nuclear industry in the United States are limited to doses of 50 mSv a year and 100 mSv every 5 years.[227]
Radiation dose units
The radiation dose reported in the gray or mGy unit is proportional to the amount of energy that the irradiated body part is expected to absorb, and the physical effect (such as DNA double strand breaks) on the cells' chemical bonds by X-ray radiation is proportional to that energy.[236]
The sievert unit is used in the report of the effective dose. The sievert unit, in the context of CT scans, does not correspond to the actual radiation dose that the scanned body part absorbs but to another radiation dose of another scenario, the whole body absorbing the other radiation dose and the other radiation dose being of a magnitude, estimated to have the same probability to induce cancer as the CT scan.[237] Thus, as is shown in the table above, the actual radiation that is absorbed by a scanned body part is often much larger than the effective dose suggests. A specific measure, termed the computed tomography dose index (CTDI), is commonly used as an estimate of the radiation absorbed dose for tissue within the scan region, and is automatically computed by medical CT scanners.[238] It is usually expressed in units of milligray (mGy). CTDI is further divided into CTDI100, CTDIw and CTDIvol. CTDI is expressed mathematically as:[239][240]
| Radiation exposure Index | Formula | |
|---|---|---|
| Computed tomography dose index | Where represent the number of slices obtained during a single axial rotation, denote the width of an individual acquired slice, and represent the radiation dose recorded at position along the primary axis of the scanner.[241] | |
| Computed tomography dose index 100 | . | |
| Computed tomography dose index weighted | is measured at center and is measured at the periphery.[241] | |
| Computed tomography dose index volume |
Another important parameter in assessing radiation dose in CT is the Dose Length Product (DLP) which is calculated as the product of CTDIvol and the scan length expressed as mGy*cm. It provides an indication of the overall dose output, taking into consideration the length of the scan. However, DLP does not account for the size of the patient and is not a direct measure of absorbed dose or effective dose.To account for variations in patient size, an additional metric, Size-Specific Dose Estimate (SSDE), is introduced. SSDE, measured in mGy, takes into account the size of the patient, providing a more estimate of absorbed dose. It focuses on accounting for variations in patient size when estimating the absorbed dose during a CT examination
The equivalent dose is the effective dose of a case, in which the whole body would actually absorb the same radiation dose, and the sievert unit is used in its report. In the case of non-uniform radiation, or radiation given to only part of the body, which is common for CT examinations, using the local equivalent dose alone would overstate the biological risks to the entire organism.[242][243][244]
Effects of radiation
Most adverse health effects of radiation exposure may be grouped in two general categories:
- deterministic effects (harmful tissue reactions) due in large part to the killing/malfunction of cells following high doses;[245]
- stochastic effects, i.e., cancer and heritable effects involving either cancer development in exposed individuals owing to mutation of somatic cells or heritable disease in their offspring owing to mutation of reproductive (germ) cells.[246]
The added lifetime risk of developing cancer by a single abdominal CT of 8 mSv is estimated to be 0.05%, or 1 one in 2,000.[247]
Because of increased susceptibility of fetuses to radiation exposure, the radiation dosage of a CT scan is an important consideration in the choice of medical imaging in pregnancy.[248][249]
Excess doses
In October, 2009, the US Food and Drug Administration (FDA) initiated an investigation of brain perfusion CT (PCT) scans, based on radiation burns caused by incorrect settings at one particular facility for this particular type of CT scan. Over 256 patients were exposed to radiations for over 18-month period. Over 40% of them lost patches of hair, and prompted the editorial to call for increased CT quality assurance programs. It was noted that "while unnecessary radiation exposure should be avoided, a medically needed CT scan obtained with appropriate acquisition parameter has benefits that outweigh the radiation risks."[227][250] Similar problems have been reported at other centers.[227] These incidents are believed to be due to human error.[227]
Cancer
The radiation used in CT scans can damage body cells, including DNA molecules, which can lead to radiation-induced cancer.[230] The radiation doses received from CT scans is variable. Compared to the lowest dose x-ray techniques, CT scans can have 100 to 1,000 times higher dose than conventional X-rays.[251] However, a lumbar spine x-ray has a similar dose as a head CT.[252] Articles in the media often exaggerate the relative dose of CT by comparing the lowest-dose x-ray techniques (chest x-ray) with the highest-dose CT techniques. In general, a routine abdominal CT has a radiation dose similar to three years of average background radiation.[253]
Studies published in 2020 on 2.5 million patients[254] and 3.2 million patients[255] have drawn attention to high cumulative doses of more than 100 mSv to patients undergoing recurrent CT scans within a short time span of 1 to 5 years.
Some experts note that CT scans are known to be "overused," and "there is distressingly little evidence of better health outcomes associated with the current high rate of scans."[251] On the other hand, a recent paper analyzing the data of patients who received high cumulative doses showed a high degree of appropriate use.[256] This creates an important issue of cancer risk to these patients. Moreover, a highly significant finding that was previously unreported is that some patients received >100 mSv dose from CT scans in a single day,[254] which counteracts existing criticisms some investigators may have on the effects of protracted versus acute exposure.
Early estimates of harm from CT are partly based on similar radiation exposures experienced by those present during the atomic bomb explosions in Japan after the Second World War and those of nuclear industry workers.[230] Some experts project that in the future, between three and five percent of all cancers would result from medical imaging.[251]
An Australian study of 10.9 million people reported that the increased incidence of cancer after CT scan exposure in this cohort was mostly due to irradiation. In this group, one in every 1,800 CT scans was followed by an excess cancer. If the lifetime risk of developing cancer is 40% then the absolute risk rises to 40.05% after a CT.[257][258]
Some studies have shown that publications indicating an increased risk of cancer from typical doses of body CT scans are plagued with serious methodological limitations and several highly improbable results,[259] concluding that no evidence indicates such low doses cause any long-term harm.[260][261][262]
One study estimated that as many as 0.4% of cancers in the United States resulted from CT scans, and that this may have increased to as much as 1.5 to 2% based on the rate of CT use in 2007.[230] Others dispute this estimate,[263] as there is no consensus that the low levels of radiation used in CT scans cause damage. Lower radiation doses are used in many cases, such as in the investigation of renal colic.[264]
A person's age plays a significant role in the subsequent risk of cancer.[227] Estimated lifetime cancer mortality risks from an abdominal CT of a one-year-old is 0.1%, or 1:1000 scans.[227] The risk for someone who is 40 years old is half that of someone who is 20 years old with substantially less risk in the elderly.[227] The International Commission on Radiological Protection estimates that the risk to a fetus being exposed to 10 mGy (a unit of radiation exposure) increases the rate of cancer before 20 years of age from 0.03% to 0.04% (for reference a CT pulmonary angiogram exposes a fetus to 4 mGy).[229] A 2012 review did not find an association between medical radiation and cancer risk in children noting however the existence of limitations in the evidences over which the review is based.[265]
CT scans can be performed with different settings for lower exposure in children with most manufacturers of CT scans as of 2007 having this function built in.[266] Furthermore, certain conditions can require children to be exposed to multiple CT scans.[230] Current evidence suggests informing parents of the risks of pediatric CT scanning.[267]
Risks vs benefits
The decision to request a CT scan involves an evaluation of potential benefits and associated risks. CT scans are invaluable for precise diagnoses, efficient treatment planning, and time-saving advantages across various medical contexts. While CT scans offer detailed anatomical insights for disease diagnosis and improved patient management, the use of ionizing radiation and contrast media raises concerns about potential harm and adverse effects. Every CT procedure must be justified, and the potential benefits should outweigh the associated risks. Preferential use of alternative, non-ionizing modalities such as ultrasound or MRI should be sought when diagnostically significant imaging can be obtained from them without compromising accuracy. In instances where a CT examination is deemed indispensable, diligent measures should be undertaken to optimize the procedure, ensuring the utilization of the minimum radiation dose essential for achieving diagnostically accurate scans. Adherence to the ALARA guidelines becomes important to mitigate unnecessary radiation exposure, prioritizing the well-being of the patient.
History
The history of X-ray computed tomography goes back to at least 1917 with the mathematical theory of the Radon transform.[268][269] In October 1963, William H. Oldendorf received a U.S. patent for a "radiant energy apparatus for investigating selected areas of interior objects obscured by dense material".[270] The first commercially viable CT scanner was invented by Godfrey Hounsfield in 1972.[271] The 1979 Nobel Prize in Physiology or Medicine was awarded jointly to South African-American physicist Allan MacLeod Cormack and British electrical engineer Godfrey Hounsfield "for the development of computer-assisted tomography".[272]
Etymology
The word "tomography" is derived from the Greek tome (slice) and graphein (to write).[273] Computed tomography was originally known as the "EMI scan" as it was developed in the early 1970s at a research branch of EMI, a company best known today for its music and recording business.[274] It was later known as computed axial tomography (CAT or CT scan) and body section röntgenography.[275] The term "CAT scan" is no longer used because current CT scans enable for multiplanar reconstructions. This makes "CT scan" the most appropriate term, which is used by radiologists in common vernacular as well as in textbooks and scientific papers.[276][277] In Medical Subject Headings (MeSH), "computed axial tomography" was used from 1977 to 1979, but the current indexing explicitly includes "X-ray" in the title.[278]
Society and culture
Campaigns
In response to increased concern by the public and the ongoing progress of best practices, the Alliance for Radiation Safety in Pediatric Imaging was formed within the Society for Pediatric Radiology. In concert with the American Society of Radiologic Technologists, the American College of Radiology and the American Association of Physicists in Medicine, the Society for Pediatric Radiology developed and launched the Image Gently Campaign which is designed to maintain high-quality imaging studies while using the lowest doses and best radiation safety practices available on pediatric patients.[279] This initiative has been endorsed and applied by a growing list of various professional medical organizations around the world and has received support and assistance from companies that manufacture equipment used in Radiology.
Following upon the success of the Image Gently campaign, the American College of Radiology, the Radiological Society of North America, the American Association of Physicists in Medicine and the American Society of Radiologic Technologists have launched a similar campaign to address this issue in the adult population called Image Wisely.[280]
The World Health Organization and International Atomic Energy Agency (IAEA) of the United Nations have also been working in this area and have ongoing projects designed to broaden best practices and lower patient radiation dose.[281]
Prevalence
| Country | Value |
|---|---|
| Template:Flagcountry | 111.49 |
| Template:Flagcountry | 64.35 |
| Template:Flagcountry | 43.68 |
| Template:Flagcountry | 42.64 |
| Template:Flagcountry | 39.72 |
| Template:Flagcountry | 39.28 |
| Template:Flagcountry | 39.13 |
| Template:Flagcountry | 38.18 |
| Template:Flagcountry | 35.13 |
| Template:Flagcountry | 34.71 |
| Template:Flagcountry | 34.22 |
| Template:Flagcountry | 28.64 |
| Template:Flagcountry | 24.51 |
| Template:Flagcountry | 24.27 |
| Template:Flagcountry | 23.33 |
| Template:Flagcountry | 19.14 |
| Template:Flagcountry | 18.59 |
| Template:Flagcountry | 18.22 |
| Template:Flagcountry | 17.36 |
| Template:Flagcountry | 17.28 |
| Template:Flagcountry | 16.88 |
| Template:Flagcountry | 16.77 |
| Template:Flagcountry | 16.69 |
| Template:Flagcountry | 15.76 |
| Template:Flagcountry | 15.28 |
| Template:Flagcountry | 15.00 |
| Template:Flagcountry | 14.77 |
| Template:Flagcountry | 13.48 |
| Template:Flagcountry | 13.00 |
| Template:Flagcountry | 9.53 |
| Template:Flagcountry | 9.19 |
| Template:Flagcountry | 5.83 |
| Template:Flagcountry | 1.24 |
Use of CT has increased dramatically over the last two decades.[53] An estimated 72 million scans were performed in the United States in 2007,[54] accounting for close to half of the total per-capita dose rate from radiologic and nuclear medicine procedures.[283] Of the CT scans, six to eleven percent are done in children,[229] an increase of seven to eightfold from 1980.[227] Similar increases have been seen in Europe and Asia.[227] In Calgary, Canada, 12.1% of people who present to the emergency with an urgent complaint received a CT scan, most commonly either of the head or of the abdomen. The percentage who received CT, however, varied markedly by the emergency physician who saw them from 1.8% to 25%.[284] In the emergency department in the United States, CT or MRI imaging is done in 15% of people who present with injuries as of 2007 (up from 6% in 1998).[285]
The increased use of CT scans has been the greatest in two fields: screening of adults (screening CT of the lung in smokers, virtual colonoscopy, CT cardiac screening, and whole-body CT in asymptomatic patients) and CT imaging of children. Shortening of the scanning time to around 1 second, eliminating the strict need for the subject to remain still or be sedated, is one of the main reasons for the large increase in the pediatric population (especially for the diagnosis of appendicitis).[230] As of 2007, in the United States a proportion of CT scans are performed unnecessarily.[266] Some estimates place this number at 30%.[229] There are a number of reasons for this including: legal concerns, financial incentives, and desire by the public.[266] For example, some healthy people avidly pay to receive full-body CT scans as screening. In that case, it is not at all clear that the benefits outweigh the risks and costs. Deciding whether and how to treat incidentalomas is complex, radiation exposure is not negligible, and the money for the scans involves opportunity cost.[266]
Additional Information
Acknowledgements
Competing interests
The authors declare that they have no competing interests.
Ethics statement
No ethics statement necessary.
References
- ↑ 1.0 1.1 Template:Cite journal
- ↑ 2.0 2.1 Template:Cite journal
- ↑ Template:Cite journal
- ↑ 4.0 4.1 Template:Cite journal
- ↑ 5.0 5.1 Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite book p 370
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite book p 371.
- ↑ Template:Cite journal
- ↑ 12.0 12.1 Template:Cite journal
- ↑ Template:Cite book p 373.
- ↑ Template:Cite book
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite book
- ↑ Template:Cite journal
- ↑ Template:Cite book
- ↑ Template:Cite book
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ 29.0 29.1 Template:Cite journal
- ↑ Template:Cite book
- ↑ Template:Cite book
- ↑ Template:Cite journal
- ↑ Template:Cite book
- ↑ Template:Cite journal
- ↑ 35.0 35.1 Template:Cite book
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ 49.0 49.1 Template:Cite journal
- ↑ 50.0 50.1 Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite book
- ↑ 53.0 53.1 Template:Cite journal
- ↑ 54.0 54.1 Template:Cite journal
- ↑ Template:Cite web
- ↑ Template:Cite journal
- ↑ Template:Cite book
- ↑ Template:Cite book
- ↑ Template:Cite book
- ↑ Template:Cite book
- ↑ Template:Cite book
- ↑ Template:Cite book
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ 65.0 65.1 Template:Cite journal
- ↑ Template:Cite book
- ↑ Template:Cite book
- ↑ Template:Cite book
- ↑ Template:Cite journal
- ↑ Template:Cite book
- ↑ Template:Cite book
- ↑ Template:Cite book
- ↑ Template:Cite journal
- ↑ Template:Cite book
- ↑ Template:Cite web
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ 78.0 78.1 78.2 Template:Cite journal
- ↑ Template:Cite journal
- ↑ 80.0 80.1 Template:Cite journal
- ↑ Template:Cite web
- ↑ 82.0 82.1 Template:Cite web
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite book
- ↑ Template:Cite web
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite book Page no. 19
- ↑ Template:Cite book
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Citation
- ↑ Template:Cite journal
- ↑ Template:Cite book
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite book
- ↑ Template:Cite book
- ↑ Template:Cite book
- ↑ Template:Cite book
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite web
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite web
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite book
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite book
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite web
- ↑ Template:Cite book
- ↑ Template:Cite book
- ↑ Template:Cite journal
- ↑ Template:Cite web
- ↑ 149.0 149.1 Template:Cite book
- ↑ 150.0 150.1 Template:Cite book
- ↑ Template:Cite web
- ↑ Template:Cite journal
- ↑ 153.0 153.1 Template:Cite journal
- ↑ Template:Cite journal
- ↑ 155.0 155.1 Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite book
- ↑ Template:Cite journal
- ↑ Template:Cite book
- ↑ Template:Cite book
- ↑ Template:Cite book
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite book
- ↑ Template:Cite book
- ↑ Template:Cite book
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite book
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ 173.0 173.1 Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite book Pg. 71
- ↑ Template:Cite book
- ↑ Template:Cite journal
- ↑ Template:Cite book Page 353
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite book
- ↑ Template:Cite web
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ 187.0 187.1 Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ 193.0 193.1 Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite book p. 373
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite book
- ↑ Template:Cite book
- ↑ Template:Cite book
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite book
- ↑ Template:Cite book
- ↑ Template:Cite book
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite web
- ↑ Template:Cite journal
- ↑ Brian R. Subach M.D., F.A.C.S et al."Reliability and accuracy of fine-cut computed tomography scans to determine the status of anterior interbody fusions with metallic cages" Template:Webarchive
- ↑ 211.0 211.1 Template:Cite journal
- ↑ 212.0 212.1 212.2 Template:Cite journal
- ↑ 213.0 213.1 Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite book
- ↑ 216.0 216.1 216.2 Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite book
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ 224.0 224.1 224.2 Template:Cite journal
- ↑ 225.0 225.1 Template:Cite web
- ↑ 226.0 226.1 226.2 226.3 226.4 226.5 Template:Cite journal
- ↑ 227.00 227.01 227.02 227.03 227.04 227.05 227.06 227.07 227.08 227.09 227.10 227.11 227.12 227.13 227.14 227.15 Template:Cite book
- ↑ 228.0 228.1 228.2 228.3 228.4 Shrimpton, P.C; Miller, H.C; Lewis, M.A; Dunn, M. Doses from Computed Tomography (CT) examinations in the UK – 2003 Review Template:Webarchive
- ↑ 229.0 229.1 229.2 229.3 229.4 Template:Cite journal
- ↑ 230.0 230.1 230.2 230.3 230.4 230.5 230.6 230.7 Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite book
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ 241.0 241.1 Template:Cite journal
- ↑ Template:Cite book
- ↑ Template:Cite web
- ↑ Template:Cite book
- ↑ Template:Cite book
- ↑ Paragraph 55 in: Template:Cite web Ann. ICRP 37 (2-4)
- ↑ Template:Cite web
- ↑ Template:Cite web
- ↑ Template:Citation
- ↑ Template:Cite journal
- ↑ 251.0 251.1 251.2 Redberg, Rita F., and Smith-Bindman, Rebecca. "We Are Giving Ourselves Cancer" Template:Webarchive, New York Times, January 30, 2014
- ↑ Template:Cite web
- ↑ Template:Cite web
- ↑ 254.0 254.1 Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite web
- ↑ Template:Cite journal
- ↑ Template:Cite web
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ 266.0 266.1 266.2 266.3 Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite book
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite book
- ↑ Template:Cite book
- ↑ Template:Cite journal
- ↑ Template:Cite web
- ↑ Template:Cite book
- ↑ Template:Cite web
- ↑ Template:Cite web
- ↑ Template:Cite web
- ↑ Template:Cite web
- ↑ Template:Cite web
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite journal