Chemicals/Reactions

Chemical reactions are associated with some changes in the chemical nature/properties of substances. For example, under specific circumstances, when hydrogen chemically reacts with oxygen, water is formed. The chemical propeties of water are quite different from its constituent elements, i.e. hydrogen and oxygen.
Chemical reactions can be classified into two main types: endothermic and exothermic reactions. An endothermic reaction is sustained only when the required quantity of heat is continuously supplied to the reaction zone and the reation absorbs heat. Exothermic reactions release heat and require removal of heat from the reaction zone for sustenance. Template:Clear
Equations

Centered above is a visual that shows how the law of conservation of atoms works: there must be the same number of atoms of each element for the reactants and the products.
Each term in the reaction equation has two parts: a coefficient indicating the number of molecules and a chemical formula for each molecule involved in the reaction.
A molecule of methane (CH4) is combined with two molecules of oxygen (O2) to produce a molecule of carbon dioxide (CO2) and two molecules of water vapor (H2O).
The physical state of the chemicals has not been included. Depending upon the conditions for which the equation records the reaction, all molecules could be gases. This may be represented as
where (g) stands for gas.
Written more generally,
Here, a = 1, A = CH4, b = 2 , B = O2, c = 1, C = CO2, d = 2, and D = H2O. Including the gaseous state of each molecule yields
The liquid methane/liquid oxygen reaction motor or engine pictured at the page top is probably preheating each right before the components enter the reaction chamber which is just above the exhaust manifold. Template:Clear
Thermodynamics
- .
- Template:Mvar: Gibbs free energy, Template:Mvar: enthalpy, Template:Mvar: temperature, Template:Mvar: entropy, Template:Math: difference (change between original and product).
At equilibrium, the Gibbs free energy is zero.
Exothermic reactions: ΔH is negative and energy is released (ΔG is negative).
Endothermic reactions: ΔS is positive, ΔT is positive, or ΔH is positive, and ΔG is positive.
- ;
ΔH° is zero at 1855 K. Above 1855 K the reaction is exothermic.
Above 1100 K the reaction is reversed.[1]
Internal energy:
- Template:Mvar: internal energy, Template:Mvar: entropy, Template:Mvar: pressure, Template:Mvar: chemical potential, Template:Mvar: number of molecules, Template:Mvar: small differential change.
Reaction kinetics
Variables:
- reactant concentrations,
- surface area for contact between reactants,
- pressure,
- temperature,
- catalyst,
- electromagnetics, and
- activation energy (Ea).
The rate v of a reaction is given by
Integration yields
k has the dimension 1/time, [A](t) is concentration at a time t and [A]0 is the initial concentration.
The Arrhenius relation for the temperature dependence is
where kB is Boltzmann's constant.
Elementary reactions

Def. a "reaction for which no reaction intermediates have been detected or need to be postulated in order to describe the chemical reaction on a molecular scale"[2] is called an elementary reaction.
"An elementary reaction is assumed to occur in a single step and to pass through a single transition state."[2]
The reaction at the top of this section shows trans-azobenzene being transformed into cis-azobenzene induced by light (hν) or heat (Δ). Trans means widely separated and cis means close by. The double arrow (⇌) indicates that the reaction is reversible. Template:Clear
Dissociation reactions
- Dissociation of a molecule AB into fragments A and B
Addition reactions
Metathesis reactions
for example,
Reaction types
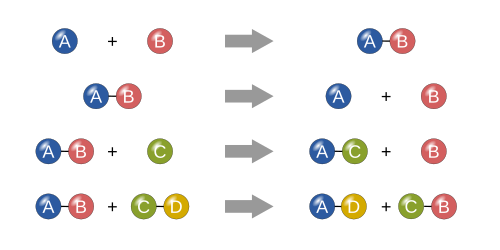
Synthesis reactions
Decomposition reactions
Single replacement reactions
- single replacement.
Double replacement reactions
- double replacement.
Oxidation and reduction

Electron transfer reactions

Sodium metal loses an electron to each fluorine atom so that
- by electron transfer.
Complexation

A neutral iron (Fe) atom yields electrons to form a complex with two C5H5 ligands. Template:Clear
Acid-base reactions
Phosphate reactions
Template:Main A phosphate reaction is a chemical reaction that leads to the transformation of one set of phosphates to another.
Def. "[a] colourless liquid, H3PO4"[3] is called phosphoric acid.
Def. "[o]rdinary phosphoric acid, H3PO4", after orthophosphoric acid, is called orthophosphoric acid.
"Orthophosphoric acid is a non-toxic, inorganic, rather weak triprotic acid, [that] is a very polar molecule ... soluble in water. ... Triprotic means that an orthophosphoric acid molecule can dissociate up to three times, giving up an H+ each time, which typically combines with a water molecule, H2O, as shown in these reactions:
- H3PO4(s) + H2O(l) <=> H3O+(aq) + H2PO4−(aq) Ka1= 7.25×10−3
- H2PO4−(aq)+ H2O(l) <=> H3O+(aq) + HPO42−(aq) Ka2= 6.31×10−8
- HPO42−(aq)+ H2O(l) <=> H3O+(aq) + PO43−(aq) Ka3= 3.98×10−13
The anion after the first dissociation, H2PO4−, is the dihydrogen phosphate anion. The anion after the second dissociation, HPO42−, is the hydrogen phosphate anion. The anion after the third dissociation, PO43−, is the phosphate or orthophosphate anion. For each of the dissociation reactions shown above, there is a separate acid dissociation constant, called Ka1, Ka2, and Ka3 given at 25 °C. Associated with these three dissociation constants are corresponding pKa1=2.12 , pKa2=7.21 , and pKa3=12.67 values at 25 °C."[4]
"For a given total acid concentration [A] = [H3PO4] + [H2PO4−] + [HPO42−] + [PO43−] ([A] is the total number of moles of pure H3PO4 which have been used to prepare 1 liter of solution), the composition of an aqueous solution of phosphoric acid can be calculated using the equilibrium equations associated with the three reactions described above together with the [H+][OH−] = 10−14 relation and the electrical neutrality equation. Possible concentrations of polyphosphoric molecules and ions is neglected. The system may be reduced to a fifth degree equation for [H+] which can be solved numerically, yielding:"[4]
| [A] (mol/L) | pH | [H3PO4]/[A] (%) | [H2PO4−]/[A] (%) | [HPO42−]/[A] (%) | [PO43−]/[A] (%) |
| 1 | 1.08 | 91.7 | 8.29 | 6.20×10−6 | 1.60×10−17 |
| 10−1 | 1.62 | 76.1 | 23.9 | 6.20×10−5 | 5.55×10−16 |
| 10−2 | 2.25 | 43.1 | 56.9 | 6.20×10−4 | 2.33×10−14 |
| 10−3 | 3.05 | 10.6 | 89.3 | 6.20×10−3 | 1.48×10−12 |
| 10−4 | 4.01 | 1.30 | 98.6 | 6.19×10−2 | 1.34×10−10 |
| 10−5 | 5.00 | 0.133 | 99.3 | 0.612 | 1.30×10−8 |
| 10−6 | 5.97 | 1.34×10−2 | 94.5 | 5.50 | 1.11×10−6 |
| 10−7 | 6.74 | 1.80×10−3 | 74.5 | 25.5 | 3.02×10−5 |
| 10−10 | 7.00 | 8.24×10−4 | 61.7 | 38.3 | 8.18×10−5 |
Phosphorylation
Template:Main Def. "the process of transferring a phosphate group from a donor to an acceptor; often catalysed by enzymes"[5] is called phosphorylation.
- D-glucose + ATP → D-glucose-6-phosphate + ADP, Gibbs free energy (ΔG°) = −16.7 kJ/mol (° indicates measurement at standard condition)
Ubiquitylation

Ubiquitination (also known as ubiquitylation or ubiquitinylation) is an enzymatic post-translational modification in which a ubiquitin protein is attached to a substrate protein, most commonly to the last amino acid of ubiquitin (glycine 76) to a lysine residue on the substrate by an isopeptide bond between the carboxyl group (COO−) of the ubiquitin's glycine and the epsilon-amino group (ε-Template:Chem) of the substrate's lysine.[6] Trypsin cleavage of a ubiquitin-conjugated substrate leaves a di-glycine "remnant" that is used to identify the site of ubiquitination.[7][8] Ubiquitin can also be bound to other sites in a protein which are electron-rich nucleophiles, termed "non-canonical ubiquitination".[9] This was first observed with the amine group of a protein's N-terminus being used for ubiquitination, rather than a lysine residue, in the protein MyoD[10] and has been observed since in 22 other proteins in multiple species,[11][12][13][14][15][16][17][18][19][20][21][22][23][24][25][26][27][12][28][29] including ubiquitin itself.[30][31] There is also increasing evidence for nonlysine residues as ubiquitination targets using non-amine groups, such as the thiol sulfhydryl group on cysteine,[26][27][32][33][34][35][36][37][38][39] and the hydroxyl group on threonine and serine.[26][27][32][38][39][40][41][42][43] The end result of this process is the addition of one ubiquitin molecule (monoubiquitination) or a chain of ubiquitin molecules (polyubiquitination) to the substrate protein.[44]
Ubiquitination requires three types of enzyme: ubiquitin-activating enzymes, ubiquitin-conjugating enzymes, and ubiquitin ligases, known as E1s, E2s, and E3s, respectively. The process consists of three main steps:
- Activation: Ubiquitin is activated in a two-step reaction by an E1 ubiquitin-activating enzyme, which is dependent on Adenosine triphosphate (ATP). The initial step involves production of a ubiquitin-adenylate intermediate. The E1 binds both ATP and ubiquitin and catalyses the acyl-adenylation of the C-terminus of the ubiquitin molecule. The second step transfers ubiquitin to an active site cysteine residue, with release of Adenosine monophosphate (AMP). This step results in a thioester linkage between the C-terminal carboxyl group of ubiquitin and the E1 cysteine thiol (sulfhydryl) group.[6][45] The human genome contains two genes that produce enzymes capable of activating ubiquitin: UBA1 and UBA6.[46]
- Conjugation: E2 ubiquitin-conjugating enzymes catalyse the transfer of ubiquitin from E1 to the active site cysteine of the E2 via a trans(thio)esterification reaction. In order to perform this reaction, the E2 binds to both activated ubiquitin and the E1 enzyme. Humans possess 35 different E2 enzymes, whereas other eukaryotic organisms have between 16 and 35. They are characterised by their highly conserved structure, known as the ubiquitin-conjugating catalytic (UBC) fold.[47]

- Ligation: E3 ubiquitin ligases catalyse the final step of the ubiquitination cascade. Most commonly, they create an isopeptide bond between a lysine of the target protein and the C-terminal glycine of ubiquitin. In general, this step requires the activity of one of the hundreds of E3s. E3 enzymes function as the substrate recognition modules of the system and are capable of interaction with both E2 and substrate. Some E3 enzymes also activate the E2 enzymes. E3 enzymes possess one of two domains: the homologous to the E6-AP carboxyl terminus (HECT) domain and the really interesting new gene RING finger domain (or the closely related U-box domain). HECT domain E3s transiently bind ubiquitin in this process (an obligate thioester intermediate is formed with the active-site cysteine of the E3), whereas RING domain E3s catalyse the direct transfer from the E2 enzyme to the substrate.[48] The anaphase-promoting complex (APC) and the SCF complex (for Skp1-Cullin-F-box protein complex) are two examples of multi-Protein subunit E3s involved in recognition and ubiquitination of specific target proteins for degradation by the proteasome.[49]
In the ubiquitination cascade, E1 can bind with many E2s, which can bind with hundreds of E3s in a hierarchical way. Having levels within the cascade allows tight regulation of the ubiquitination machinery.[50] Other ubiquitin-like proteins (UBLs) are also modified via the E1–E2–E3 cascade, although variations in these systems do exist.[51]
E4 enzymes, or ubiquitin-chain elongation factors, are capable of adding pre-formed polyubiquitin chains to substrate proteins.[52] For example, multiple monoubiquitylation of the tumor suppressor p53 by Mdm2[53] can be followed by addition of a polyubiquitin chain using P300-CBP coactivator family (p300 and CBP).[54][55] Template:Clear
Solubility

Under certain conditions, the equilibrium solubility can be exceeded to give a so-called supersaturated solution, which is metastable.[56]
According to the International Union of Pure and Applied Chemistry (IUPAC) definition,[57] solubility is the analytical composition of a saturated solution expressed as a proportion of a designated solute in a designated solvent. Solubility may be stated in various units of concentration such as molarity, molality, mole fraction, mole ratio, mass (solute) per volume (solvent) and other units.
The extent of solubility ranges widely, from infinitely soluble (without limit) (fully miscible[58]) such as ethanol in water, to poorly soluble, such as silver chloride in water. The term insoluble is often applied to poorly or very poorly soluble compounds. A number of other descriptive terms are also used to qualify the extent of solubility for a given application. For example, U.S. Pharmacopoeia gives the following terms:
| Term | Mass parts of solvent required to dissolve 1 mass part of solute[59] |
|---|---|
| Very soluble | <1 |
| Freely soluble | 1 to 10 |
| Soluble | 10 to 30 |
| Sparingly soluble | 30 to 100 |
| Slightly soluble | 100 to 1000 |
| Very slightly soluble | 1000 to 10,000 |
| Practically insoluble or insoluble | ≥ 10,000 |
The thresholds to describe something as insoluble, or similar terms, may depend on the application: one source states that substances are described as "insoluble" when their solubility is less than 0.1 g per 100 mL of solvent.[60]
Solubility (metastable, at concentrations approaching saturation) also depends on the physical size of the crystal or droplet of solute (or, strictly speaking, on the specific surface area or molar surface area of the solute).[61]
As the temperature is raised, gases usually become less soluble in water (to minimum, which is below 120 °C for most permanent gases[62]), but more soluble in organic solvents.[63]
The chart shows solubility curves for some typical solid inorganic salts (temperature is in degrees Celsius i.e. kelvins minus 273).[64] Many salts behave like barium nitrate and disodium hydrogen arsenate, and show a large increase in solubility with temperature. Some solutes (e.g., sodium chloride in water) exhibit solubility that is fairly independent of temperature. A few, such as calcium sulfate (gypsum) and cerium(III) sulfate, become less soluble in water as temperature increases.[65]
A few exceptions exist, such as certain cyclodextrins.[66]
For condensed phases (solids and liquids), the pressure dependence of solubility is typically weak and usually neglected in practice. Assuming an ideal solution, the dependence can be quantified as:
where the index i iterates the components, Ni is the mole fraction of the ith component in the solution, P is the pressure, the index T refers to constant temperature, Vi,aq is the partial molar volume of the ith component in the solution, Vi,cr is the partial molar volume of the ith component in the dissolving solid, and R is the universal gas constant.[67]
In the presence of small bubbles, the solubility of the gas does not depend on the bubble radius in any other way than through the effect of the radius on pressure (i.e., the solubility of gas in the liquid in contact with small bubbles is increased due to pressure increase by Δp = 2γ/r; see Young–Laplace equation).[68]
A popular aphorism used for predicting solubility is "like dissolves like" also expressed in the Latin language as "Similia similibus solventur".[69] This statement indicates that a solute will dissolve best in a solvent that has a similar chemical structure to itself. This view is simplistic, but it is a useful rule of thumb. The overall solvation capacity of a solvent depends primarily on its polarity.[70] For example, a very polar (hydrophilic) solute such as urea is very soluble in highly polar water, less soluble in fairly polar methanol, and practically insoluble in non-polar solvents such as benzene. In contrast, a non-polar or lipophilic solute such as naphthalene is insoluble in water, fairly soluble in methanol, and highly soluble in non-polar benzene.[71]
The rate of dissolution can be often expressed by the Noyes–Whitney equation or the Nernst and Brunner equation[72] of the form:
where:
- m = mass of dissolved material
- t = time
- A = surface area of the interface between the dissolving substance and the solvent
- D = diffusion coefficient
- d = thickness of the boundary layer of the solvent at the surface of the dissolving substance
- Cs = mass concentration of the substance on the surface
- Cb = mass concentration of the substance in the bulk of the solvent
Some substances may dissolve incongruently, whereby the composition of the solute in solution does not match that of the solid and is accompanied by alteration of the "primary solid" and possibly formation of a secondary solid phase, but in general, some primary solid also remains and a complex solubility equilibrium establishes; e.g. dissolution of albite may result in formation of gibbsite.[73]
- NaAlSi3O8(s) + H+ + 7H2O ⇌ Na+ + Al(OH)3(s) + 3H4SiO4.
The solubility of albite is expected to depend on the solid-to-solvent ratio, where it results in formation of metamorphic rocks. Template:Clear
Precipitation

The diagram on the right shows the differences between a compound, a precipitate, a supernate, and a suspension. Template:Clear
Gas-solid interface reactions
Photochemical reactions

Photoexcitation of a carbonyl group adds it to an olefin producing an oxetane. Template:Clear
Solid-state reactions
Template:Main These reactions are usually governed by interdiffusion rates.
Hypotheses
- Chemical reactions often occur in small steps.
See also
Template:Div col Template:Div col end
References
External links
Template:Chemistry resourcesTemplate:Sisterlinks
- ↑ Template:Cite web
- ↑ 2.0 2.1 Template:Cite web
- ↑ Template:Cite web
- ↑ 4.0 4.1 Template:Cite web
- ↑ Template:Cite web
- ↑ 6.0 6.1 Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ 12.0 12.1 Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ 26.0 26.1 26.2 Template:Cite journal
- ↑ 27.0 27.1 27.2 Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ 32.0 32.1 Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ 38.0 38.1 Template:Cite journal
- ↑ 39.0 39.1 Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite journal
- ↑ Template:Cite web
- ↑ Template:Cite book
- ↑ Template:Cite book
- ↑ "Pharmacopeia of the United States of America, 32nd revision, and the National Formulary, 27th edition," 2009, pp.1 to 12.
- ↑ Template:Cite web
- ↑ Template:Cite book
- ↑ Template:Cite book
- ↑ John W. Hill, Ralph H. Petrucci, General Chemistry, 2nd edition, Prentice Hall, 1999.
- ↑ Template:Cite book
- ↑ Template:Cite web
- ↑ Template:Cite journal
- ↑ Template:Cite book
- ↑ Template:Cite journal
- ↑ Template:Cite book
- ↑ The solvent polarity is defined as its solvation power according to Reichardt
- ↑ Template:Cite book
- ↑ Template:Cite journal
- ↑ Template:Cite book